The flowability of a fine-grained bulk solid depends largely on the adhesive forces between individual particles [Dietmar Schulze: Powders and Bulk Solids Behavior]. These adhesive forces are due to van der Waals and electrostatic forces. In the presence of ambient humidity, water adsorption layers and liquid bridges usually are more important. Water adsorption layers can enhance the van der Waals forces by smoothing-out the surface roughness of the particles resulting in a reduction of the actual distance between them. Also, chemical species can dissolve on the surface of the particles and react, become rubbery and plasticized. As the particles softens, the adhesion forces are enhanced due to plastic deformation increasing particle-particle contact. With small amounts of “free” water, e.g., due to capillary condensation, liquid bridges begin to form binding the particles due to capillary forces and surface tension. Under some conditions, moisture-caking could occur causing a significant reduction of the bulk solids flowability.
Therefore, an understanding of the behavior of a bulk solid in the presence of ambient humidity can be very important when measuring flowability. The moisture sorption isotherm is a valuable tool for this purpose since it provides the relationship between a bulk solid equilibrium moisture content and water activity (or relative humidity RH divided by 100) at a constant temperature. Foodstuff, salts, chemicals, etc experience different interactions with water; thus each substance has its own unique moisture isotherm. In general, the simplest approach for creating a moisture sorption isotherm is to put dried samples into controlled humidity chambers at constant temperature and measure the weight gain with time until equilibrium occurs for each chamber.

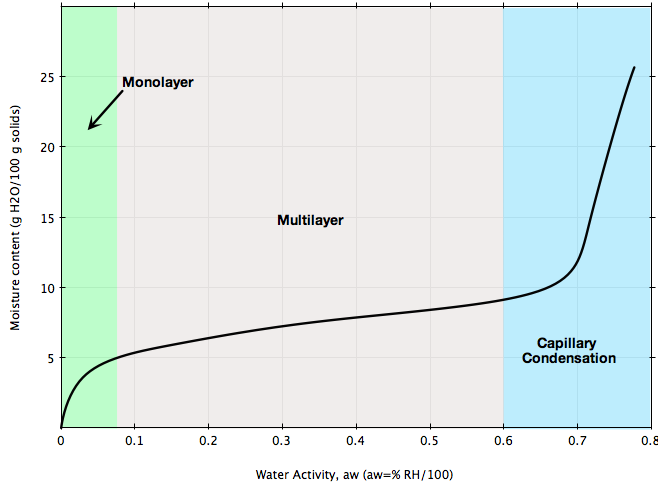
Two common types of moisture sorption isotherm are depicted in Figures A and B.The isotherm in Figure A is typical of most foods such as cereals.: at low relative humidities, adsorption takes place in mono-molecular layers; at higher relative humidity levels, multilayer adsorption takes place until capillary condensation starts.
The isotherm in Figure B represents the behavior of a pure crystalline substance, e.g., sucrose, salt: generally, crystalline substances show very little moisture gain until the water activity goes above the point where water begins to dissolve the crystal surface. This is sometimes referred to as the deliquescent point.
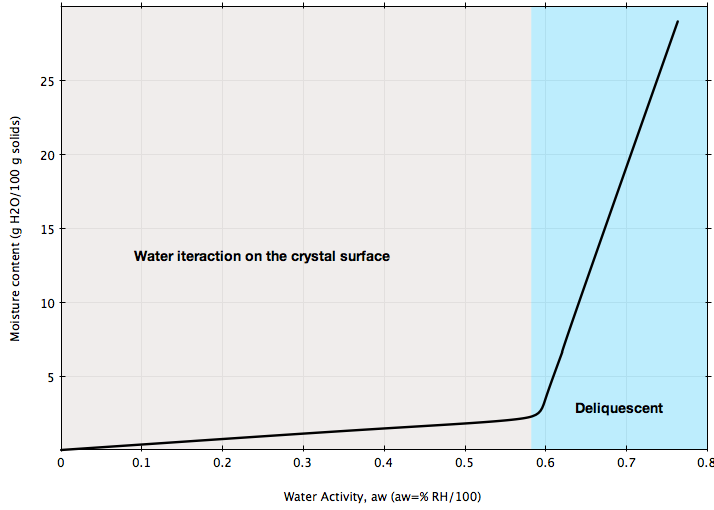
If your bulk solid is stored at ambient conditions representing the blue region in Figures A or B, chances are high that its flowability will be greatly affected and flow problems may arise. Or, if you are planning to install a handling system for a material that can be sensible to ambient moisture as illustrated in the figures, make sure the design of that system is based on the flow properties of the material measured at ambient humidity conditions representing your process.



